Dry eye is an eye disease so common that research shows that 90% of the population in large cities suffers from it, so it is easy to take it lightly. However, if dry eye syndrome is left untreated and left untreated, it can cause secondary eye diseases such as corneal ulcers, vision loss, and even blindness, as well as cataracts, glaucoma, and retinal disorders.
It is a disease that requires active treatment and consistent management.
Seodong Medical’s ‘NURIEYE’ is leading the treatment device market in this field. Until now, many patients were unaware of the seriousness of dry eye syndrome, and it was neglected due to lack of proper treatment. However, with the advent of ‘NURIEYE’, dry eye syndrome is no longer a disease that is difficult to treat, and has become ‘a disease that anyone can easily treat.
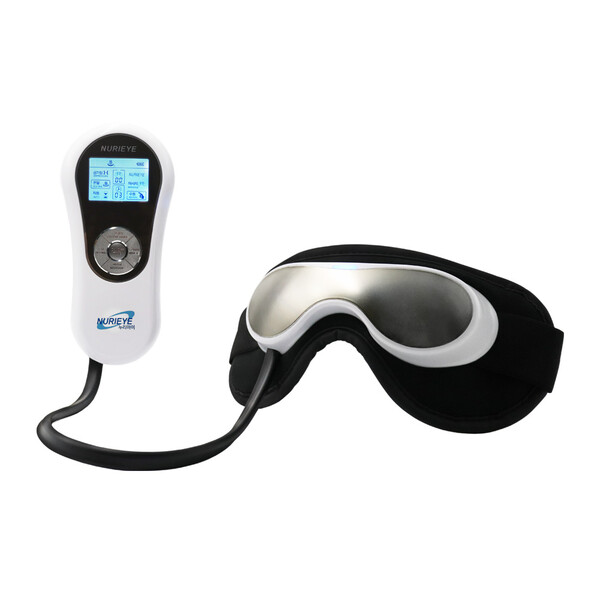
NURIEYE 5800 product image
■ Developed ‘dry eye treatment device’ for 28 years
‘Nuri Eye’, which is attracting attention as a representative medical device for treating dry eye syndrome, was developed by Seodong Medical (CEO Kim Chang-on). Founded in 1996, we have been digging the well of developing dry eye treatment devices for 28 years. Through this, we have contributed to improving the people’s eye health and have increased brand awareness by continuously exhibiting at domestic and overseas exhibitions and fairs.
In particular, Seodong Medical’s representative medical device for treating dry eye syndrome, NURIEYE-5800, received government approval for clinical trials and was tested on 2 dry eye patients at clinical trial centers at two university hospitals designated by the Ministry of Food and Drug Safety. Safety and effectiveness were confirmed and the technology was recognized. As a result of the clinical trial, dry eye syndrome was cured, and according to the results of intraocular pressure measurement, NURIEYE’s characteristic of massaging the area around the eyes led to a reduction in intraocular pressure.
■ Recognized non-reimbursed treatment at eye hospitals using new medical technology
A paper was published in the January 2014 issue of the British Journal of Ophthal mology, a British SCI journal. In November 1, the safety and effectiveness evaluation results of the new medical technology were announced, and non-reimbursed treatment has been provided at eye hospitals nationwide since July 2014. .
In addition, it has achieved achievements such as being selected as a world-class product by the Ministry of Trade, Industry and Energy, receiving recognition for new medical technology evaluation from the Ministry of Health and Welfare, GMP, Innobiz, corporate research institute, venture company certification, FDA registration, CE, UKCA certification, and winning the Seoul International Invention Award.
NURIEYE products also underwent safety inspections for sanitary sheets used as consumables and pouches that come in direct contact with the skin, and electromagnetic wave certification for the adapter was also completed.
In addition, the electromagnetic waves are ‘0’ and it can be safely used with confidence as it uses a carbon fiber heating element that changes electrical energy into far-infrared rays that are beneficial to the human body. NURIEYE-5800 is a trustworthy product registered with the FDA in the United States and sold for welfare purposes. To date, Nuriai is the only dry eye treatment medical device that has been verified for safety and effectiveness.

NURIEYE 5800-Model Cut
■ Treats dry eye syndrome by improving natural tear circulation
‘NURIEYE-5800’ massages the eyes for 15 minutes with 5 types of vibration massage, 15 levels of air pressure massage, and high and low temperature heat massage.
Vibration massage and pneumatic massage gently relax the skin muscles around the eyes to facilitate circulation of nerves, blood vessels, and tears. Warm heat massage melts foreign substances stuck or accumulated in the eye and hardened fatty secretions from the meibomian glands inside the eyelids. Helps clean tear circulation. This can treat dry eye syndrome and provide an improvement in the treatment of eye-related diseases such as cataracts and glaucoma.
NURIEYE can be used comfortably regardless of space or time, whether lying down, sitting, on an airplane or in a car while traveling.
■ Based on safety and effectiveness, it is also used for therapeutic purposes in hospitals
As the saying goes, if the body is worth a thousand nyang, the eyes are 900, so the eyes are part of the brain and are the most sensitive and important organ in the human body. For your precious eyes, you must use proven products.
NURIEYE received permission from the Ministry of Food and Drug Safety and conducted clinical trials on 144 patients with dry eye syndrome in accordance with the Ministry’s notice. As a result, it was proven to not only treat dry eye syndrome but also reduce intraocular pressure, making it a product that has been verified for safety and effectiveness. In addition, it is a proven product that has been evaluated as a new medical technology and is being used by many ophthalmologists for the purpose of treating dry eye syndrome on a non-reimbursed basis.
Meanwhile, NURIEYE is a product developed and produced in Korea by a designated corporate research center, and has the advantage of convenient services such as after-sales service.
Source : Medical Newspaper (http://www.bosa.co.kr/news/articleView.html?idxno=2218452)


